Research and Development
| Therapeutic Area | Project Code | Target | Indications | Nonclinical |
Pre-IND |
IND | Phase I | Phase II | Phase III |
Tumor 
|
HOT-1010 | VEGF | Metastatic colorectal cancer Advanced metastatic or recurrent NSCLC | ||||||
| HB002.1T | VEGF | Solid tumors | |||||||
| HOT-1030 | CD137 | Solid tumors | |||||||
| HB0030 | TIGIT | Solid tumors | |||||||
| HB0025 | VEGF/PD-L1 | Solid tumors | |||||||
| HB0028 | TGF-β/PD-L1 | Solid tumor | |||||||
| HB0036 | TIGIT/PD-L1 | Solid tumors | |||||||
| HB0045 | CD73 cocktail | Solid tumors | |||||||
| HB0052 | CD73-ADC | Solid tumors | |||||||
Autoimmune 
|
HB0056 | TSLP/IL-11 | Asthma / AD /IPF /COPD | ||||||
| HB0043 | IL-17A/IL-36R | HS /LN /CKD /AD | |||||||
| HOT-3010 | TNF-α | Rheumatoid arthritis Ankylosing spondylitis Moderate/severe plaque psoriasis in adults | |||||||
| HB0017 | IL-17 | Moderate,severe plaque psoriasis / Psoriatic arthritis Ankylosing spondylitis | |||||||
| HB0034 | IL-36R | Pustular psoriasis Systemic lupus erythematosus Ulcerative colitis |
|||||||
Ophthalmic 
|
HB002.1M | VEGF | AMD/DME | ||||||
HB0025 A Bispecific Fusion Protein Targeting PDL-1 and VEGF
HB0025 joins VEGFR1 fragment and anti-PD-L1 monoclonal antibody so that it can target the PD-L1 and VEGF with high specificity. Anti-PD-L1 monoclonal antibody relieves the immunosuppressive effect mediated by PD-1/PD-L1 pathway, activates cytotoxic T lymphocytes, thereby inhibiting tumor growth; VEGFR1 fragment as a trap for VEGF can inhibit the proliferation of vascular endothelial cells and the formation of new blood vessels, and improves the infiltration of cytotoxic T lymphocytes in the tumor microenvironment. Pre-clinical studies have shown that HB0025 has a synergistic effect on the blocking of the above two signal pathways, and its curative effect is significantly better than the single drug and the combination of two single drugs. Therefore, HB0025 has great therapeutic potential for a variety of advanced solid tumors, especially advanced solid tumors that are ineffective or resistant to PD-1/PD-L1 or VEGF/VEGFR inhibitor treatment.
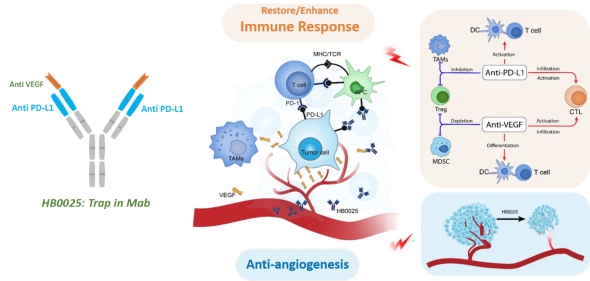
Schematic diagram of HB0025 mode of action
At present, there is no drug that simultaneously targets the two signaling pathways of PD-1/PD-L1 and VEGF/VEGFR on the market. The combination therapy of Atezolizumab (anti-PD-L1 monoclonal antibody) and Bevacizumab (anti-VEGF monoclonal antibody) developed by Roche has been approved by the FDA and the National Medical Products Administration for the treatment of unresectable hepatocellular carcinoma. Atezolizumab combined with Bevacizumab and chemotherapy have also been approved by the FDA as the first-line treatment of metastatic non-squamous non-small cell lung cancer without EGFR or ALK mutations. HB0025 has been approved for clinical trials in China and the United States. The Phase I clinical study has been launched in the United States in early 2021.